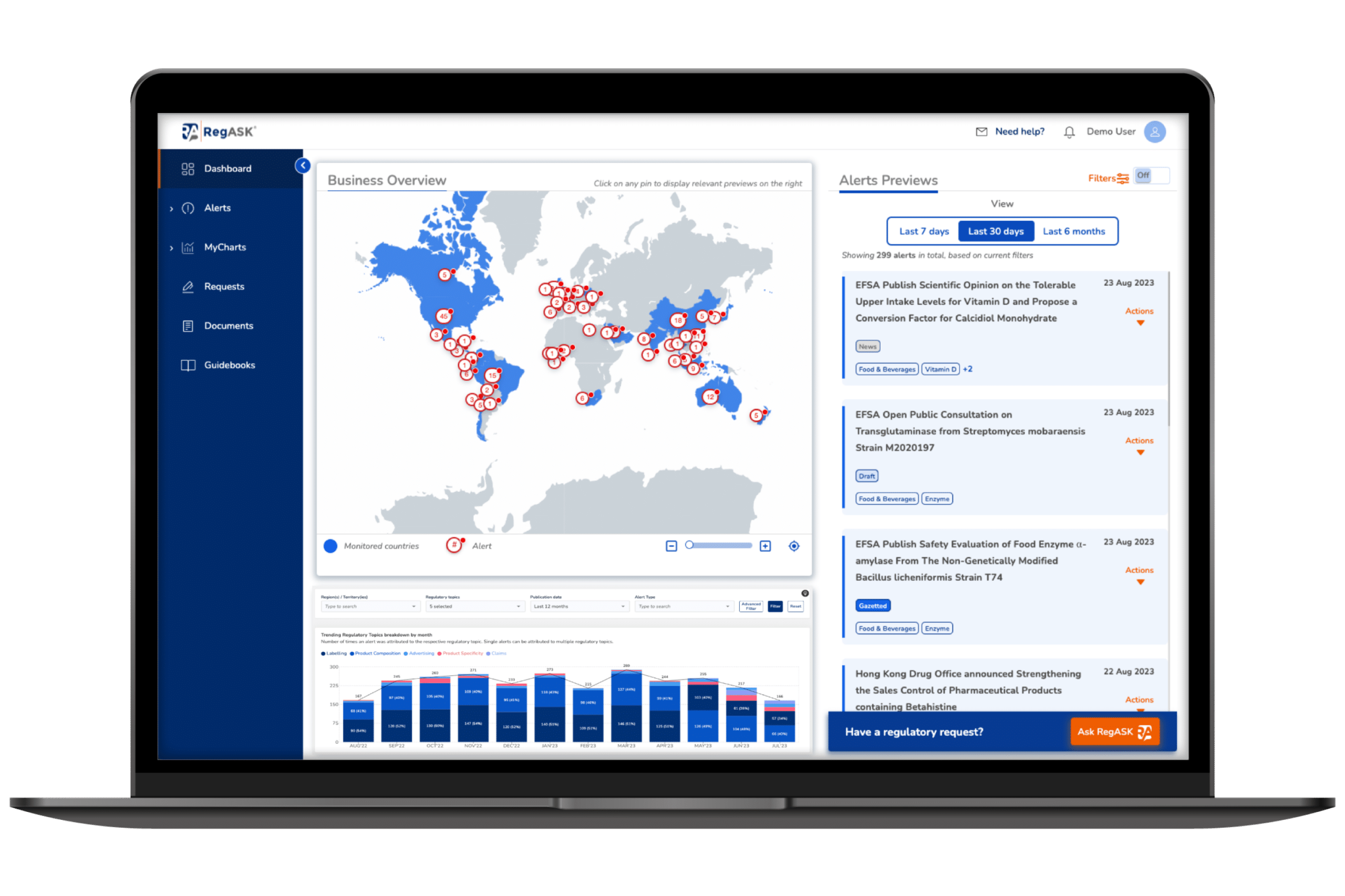
Life Science
Transform compliance into a strategic asset.
Navigate seamlessly through increasing regulatory mandates and technological advancement.
Consumer Packaged Goods
Be a catalyst for business growth. Confront industry challenges head-on – from regulatory dynamics to market demands. Elevate efficiency, mitigate risks, and get ahead in the dynamic landscape.
Learn more