El TGA is making changes to the maximum recommended daily dose of vitamin B6 permitted in Listed (low risk) medicines such as Complementary Medicines. Currently, Vitamin B6 in human medicines is restricted to use in Schedule 4 (Prescription Only) medicines except:
- In oral preparations containing 200 mg or less but more than 50 mg of pyridoxine, pyridoxal, or pyridoxamine per recommended daily dose when compliant with the requirements of the Required Advisory Statements for Medicine Labels (RASML*); or
- In oral preparations containing 50 mg or less of pyridoxine, pyridoxal, or pyridoxamine per recommended daily dose.
*Note: The RASML requires the (VITB6SX) statement on labels ‘WARNING – Stop taking this medication if you experience tingling, burning, or numbness and see your healthcare practitioner as soon as possible. [Contains vitamin B6].’ To be clear, products that contain 50 mg or less per recommended daily dose do not require the label warning.
Following a review of adverse events associated with long-term use of Vitamin B6 supplements, and based on the Nutrient Reference Values for Australia and New Zealand for vitamin B6 is set at 50 mg pyridoxine/day for adults and much lower for infants, the TGA has decided to amend the entries in the Permissible Ingredients Determination as follows:
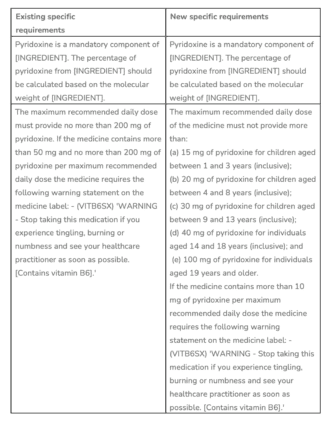
‘INGREDIENT’ = Pyridoxal 5-phosphate, Pyridoxal 5-phosphate monohydrate, Pyridoxine hydrochloride. These are the permitted forms for listed medicines.
The final changes will commence on 1 March 2022 with a one-year transition period to allow sponsors (MA holders) to ensure product compliance ending on 1 March 2023.
Contact RegASK to know more about regulation changes in Australia.
Contacte con RegASK para más detalles Leer más
Suscríbete a las últimas novedades regulatorias
Boletines informativos seleccionados
Información relevante de la industria
Acceda a información de expertos

