The Natural Cosmetics Act (NCA), recently introduced in the United States Congress, outlines a potential reconfiguration of regulations governing personal care products, primarily cosmetics.
Cosmetics regulations in the U.S. have not changed since the FD&C Act passed in 1938 – this proposed law, if passed, would dramatically change the way manufacturers label and market their products.
Why the Need for Change?
On average, women use 12 personal care products a day, exposing themselves to 168 chemical ingredients; men use six, and are exposed to 85 unique chemicals. This alarming statistic illustrates why consumers are looking for brands that use fewer chemicals, however, it’s currently difficult for consumers to distinguish what products are cleaner than others.
The current regulations do not require cosmetic labeling to gain FDA approval before market entry, meaning any brand can use the word “natural” on their packaging. The FDA doesn’t hold the same authority over cosmetics as it does with drugs or medical devices, meaning companies are left to self-regulate. At times, products (with natural claims) are sold on the market that contain less-than-favorable ingredients. The proposed legislation aims to prevent harmful chemicals from finding their way into personal care products.
The Personal Care Products council, a group representing 600 well-known cosmetic companies, has voiced support for the proposed legislation, saying the bill will provide the FDA more authority and “further strengthen consumer confidence” in cosmetic products.
Defining “Natural” and “Naturally Derived”
The goal of the NCA is to require cosmetic companies to provide more transparency regarding the ingredients found in their products. The bill would define the term “natural” and require verification that the natural ingredients used are authentic. As it stands under current regulation, falsely labeling a product with a “natural” claim is not considered misbranding, as no definition of “natural” nor “naturally derived” exists.
Here’s an easy breakdown of the specifics in the proposed legislation:
- Cosmetics sold, labeled, or represented as “natural” must contain at least 70 percent natural substances, excluding water, to house the claim.
- Suppliers must submit results of Carbon-14 testing to manufacturers (verifies the authenticity of natural ingredients) before market entry.
- FDA authorized to issue a cease distribution order, place a public notice on the FDA website, and voluntarily recall any product deemed misbranded or adulterated.
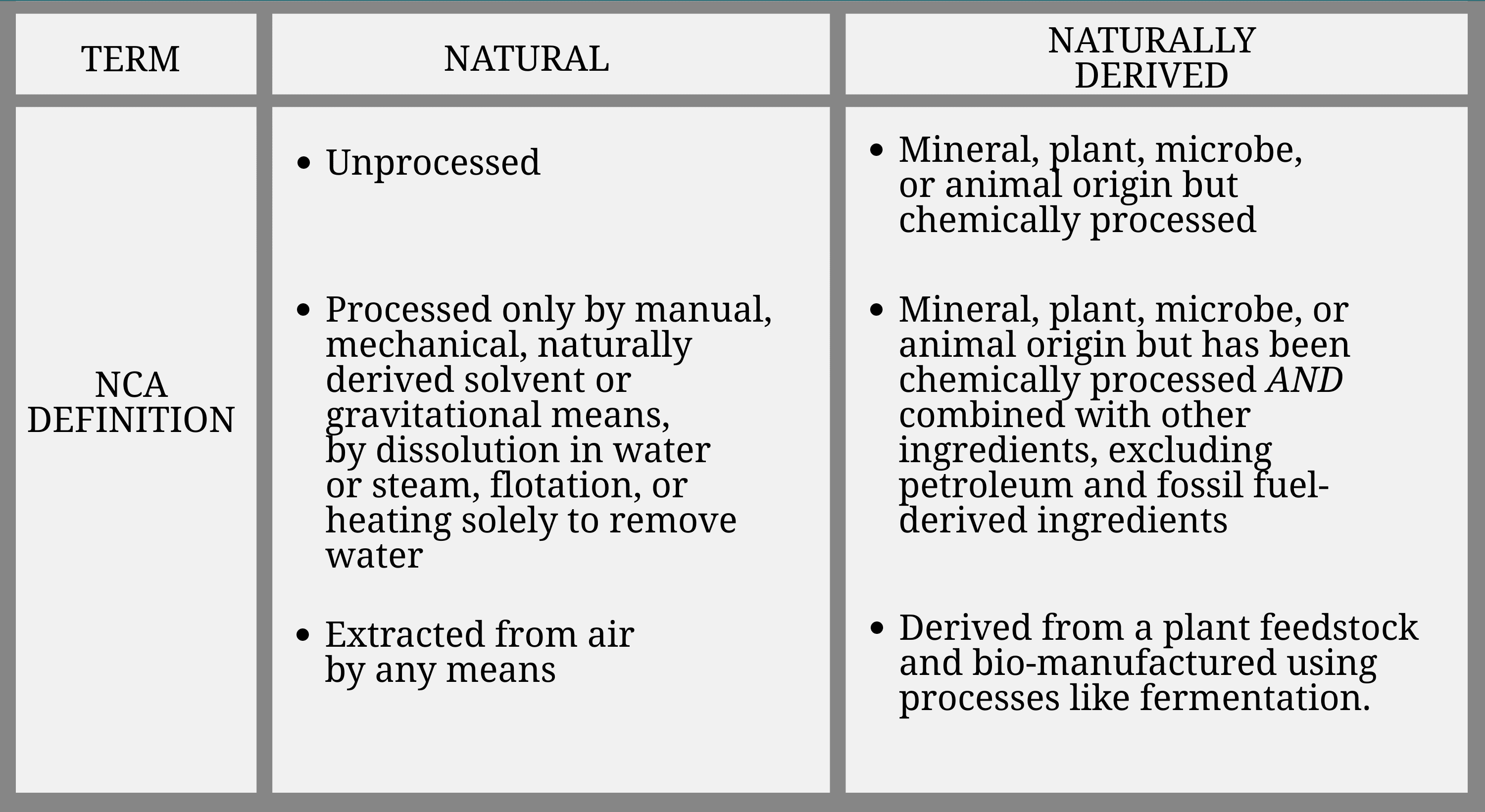
The following table details the definitions for both “natural” and “naturally derived” as outlined in the NCA:
What Does This Mean for Cosmetic Companies?
This regulatory shift would take claim regulation out of the hands of the producer, ceding the responsibility to the FDA. Should it be passed, producers will have only two years from the date of its enactment to comply with the legislation.
It also more closely aligns U.S. regulations with European legislation, meaning companies would need to provide verifiable evidence to substantiate their claims. When compared to the existing EC regulations, the NCA shares similarities in that cosmetics claims must be backed by testing. In the U.S., Carbon-14 analysis would be used to verify “natural claims.” This type of testing helps to determine if a product contains synthetic properties, suggesting it may not be truly “natural.”
Hopes are that the NCA would allow consumers to confidently determine which products are made with safe, natural ingredients, but we’ll have to wait to see if Congress passes the bill, first.