This article is based on insights generated by RegGenius, RegASK AI-driven regulatory intelligence tool.
在 April 2023, Denmark became the first European nation to officially ban ashwagandha, immediately requiring all products containing the popular adaptogenic herb to be removed from store shelves. This wasn’t just another routine regulatory update. Within months, what started as a single-country decision would trigger a domino effect across 欧洲, forcing supplement companies to completely rethink their strategies.
Between 2023 and 2025, authorities focused not only on ashwagandha but also on kratom, Tongkat Ali, and green tea extracts reflecting heightened safety concerns and stricter consumer protection standards. For companies built on adaptogenic herbs [1] and traditional botanicals, understanding these regulatory shifts isn’t just business intelligence; it’s a survival strategy.
This blog examines the current herbal supplement regulatory landscape, explores which botanicals are at risk, and provides actionable strategies for maintaining compliance in an increasingly complex global market.
The Essentials
- Europe is imposing bans and restrictions on herbal supplements like ashwagandha, kratom, and green tea extracts due to safety concerns.
- Health Supplement companies are facing product recalls, reformulation costs, and complex compliance challenges across different markets.
- Industry experts emphasize the need for proactive regulatory intelligence, diversified formulations, and robust safety documentation to stay compliant.
Table of Contents
- Which Herbal Supplements Are Under Increased Scrutiny and Why?
- The Case of Ashwagandha: Europe’s Fragmented Response
- How These Bans Affect Supplement Businesses
- What Steps Can You Take to Stay Compliant?
- What to Expect Beyond 2025
- How RegASK helps navigate Global Regulatory Complexity
Which Herbal Supplements Are Under Increased Scrutiny and Why?
The herbal supplement industry is facing unprecedented regulatory pressure as authorities worldwide intensify oversight of products that may pose safety risks. This scrutiny spans multiple issues, from traditional botanicals to products contaminated with undeclared pharmaceutical ingredients. Understanding which supplements are under regulatory review is crucial for manufacturers, distributors, and consumers navigating this evolving landscape.
1. Botanicals Under Regulatory Review in the EU
In the EU, several botanicals are under review for their classification, safety, and permissible use in food supplements:
- Ashwagandha (Withania somnifera)
- Melatonin
- Kratom (Mitragyna speciosa)
- Some Traditional Chinese Medicine (TCM) products
2. Menopause Supplements under Regulatory Lens
Many women turn to herbal supplements to manage menopause symptoms. The following ingredients, which are under scrutiny, are commonly found in menopause supplements:
- Chaste tree berry (Vitex agnus-castus)
- Red clover (Trifolium pratense)
- Black cohosh (Actaea racemosa)
- Dong quai (Angelica sinensis)
- Maca (Lepidium meyenii)
Experts have raised concerns about their safety, efficacy, potential for liver harm, and negative interactions with other drugs. As a result, increased regulatory oversight and growing consumer skepticism are expected for these products.
3. Supplements Containing Banned Ingredients
Regulatory authorities, such as the Singapore Health Sciences Authority (HSA), 美国食品药品管理局 (FDA) 和 加拿大卫生部, have issued multiple advisories on herbal and health supplements found to contain undeclared or prohibited ingredients, including: [2] [3]
- Steroids (e.g., dexamethasone, prednisolone)
- Sibutramine (banned weight loss drug)
- Phosphodiesterase type 5 (PDE-5) inhibitors (e.g., sildenafil, tadalafil)
- Diclofenac
- 对苯二酚
- Fluoxetine
- Prasterone (DHEA)
- Methocarbamol
- Lidocaine
- Phenolphthalein
- Sennosides
These ingredients have been linked to serious adverse effects, including cardiovascular events, liver damage, kidney disorders, and other health risks. Products containing these substances are subject to recalls, bans, and enforcement actions. Such products pose significant health risks due to undisclosed ingredients and potential drug interactions.
4. Supplements Adulterated with Undeclared Pharmaceuticals
Global regulators are cracking down on supplements containing undeclared pharmaceutical ingredients, which are often disguised as “natural” products for sexual enhancement, weight loss, or energy. Israeli Ministry of Health has issued warnings about hidden substances like sildenafil, tadalafil, 和 levodopa, which can cause serious health risks when taken unknowingly.
5. Contaminated and Counterfeit Supplements
Beyond hidden drugs, supplements contaminated with heavy metals, microbial toxins, or sold as counterfeits remain a major global concern. Regulators are stepping up product testing and import checks to protect consumers and penalize non-compliant brands.
The Case of Ashwagandha: Europe’s Fragmented Response
Ashwagandha (Withania somnifera) has become one of the most controversial botanicals in Europe’s supplement market. Driven by consumer interest in stress relief and natural health, the European ashwagandha market’s volume surged by 10.9% between 2019 and 2022, with continued double digit growth forecast through 2030. [5]
Why Ashwagandha Became a Regulatory Target
Ashwagandha’s popularity proved to be its downfall. As millions turned to ashwagandha for stress relief and anxiety management, regulatory bodies grew concerned about its complex chemical profile. Unlike single molecule drugs, ashwagandha root extract contains dozens of active compounds, including withanolides, making safety assessment challenging. The regulatory tipping point came when adverse event reports surfaced, particularly cases of liver toxicity. While most involved with contaminated products or extreme doses, the damage was done, and European regulators moved into action mode.
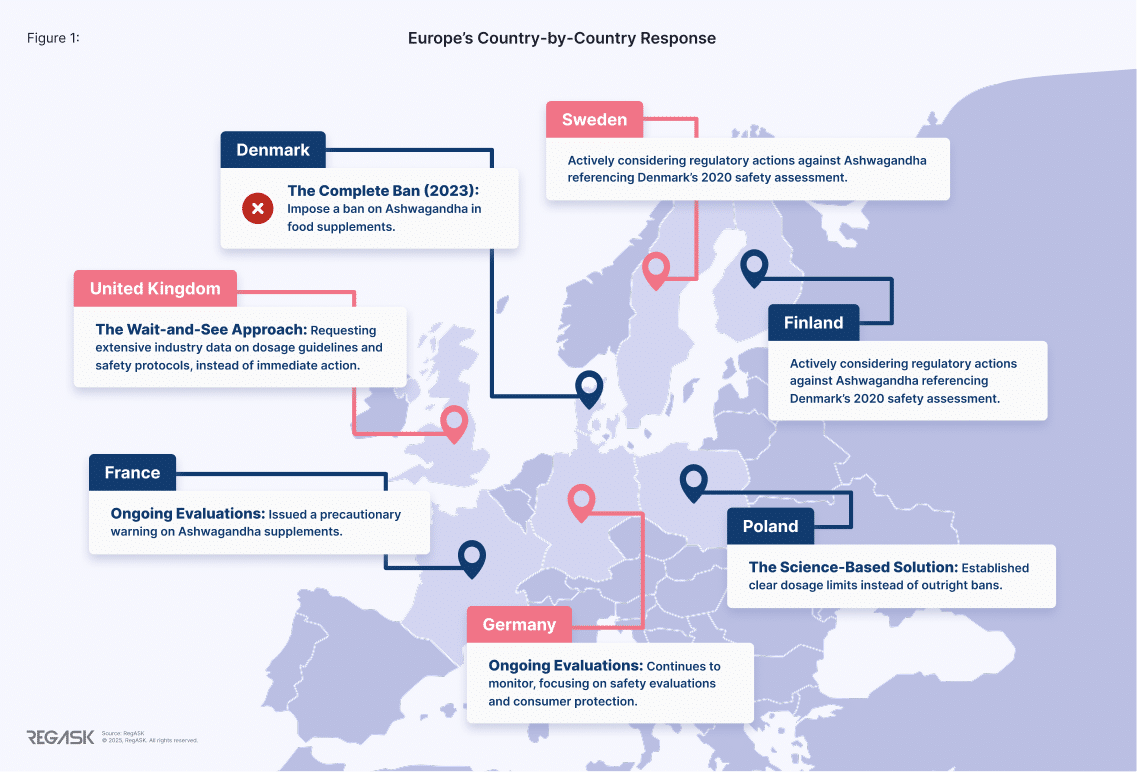
Europe’s Country-by-Country Response
Denmark: The Complete Ban (2023)
Denmark became the first EU country to impose a ban on ashwagandha in food supplements, following a 2020 risk assessment by the Technical University of Denmark (DTU) that raised concerns about hormonal disruption and potential reproductive risks. [6] [7]
The Danish Veterinary and Food Administration (DVFA) enforced the ban rapidly, requiring products to be withdrawn from the market. This decision had a major impact on supplement companies operating in the Nordic region, with industry groups reporting significant financial losses.[8]
United Kingdom: The Wait-and-See Approach
The UK Food Standards Agency chose a comprehensive review instead of immediate action, requesting extensive industry data on dosage guidelines and safety protocols. While more measured, this created regulatory limbo for manufacturers unable to make long term decisions.
Poland: The Science-Based Solution
Poland established clear dosage limits instead of outright bans:
Nordic Alliance: Sweden and Finland
Sweden and Finland are actively considering regulatory actions against ashwagandha, primarily referencing Denmark’s 2020 safety assessment by the Technical University of Denmark (DTU) as justification.
France and Germany: Ongoing Evaluations [11] [12]
In 2024, France’s food safety agency ANSES issued a precautionary warning on ashwagandha supplements, advising vulnerable groups—including pregnant or breastfeeding women, minors, and individuals with thyroid, liver, or heart conditions—to avoid use due to reported adverse effects and limited safety data. This has prompted stricter labeling requirements and calls for enhanced regulatory oversight within France’s health supplement market.
Meanwhile, Germany continues to monitor herbal supplements like ashwagandha under its Federal Institute for Risk Assessment (BfR), focusing on safety evaluations and consumer protection, though no formal bans have been enacted.
How These Bans Affect Supplement Businesses
Financial Impact Across the Supply Chain
The regulatory uncertainty surrounding ashwagandha disrupted the supply chain at multiple levels. Raw material suppliers faced a sharp decline in orders, particularly in markets enforcing restrictions.
Contract manufacturers were forced to reformulate products—often incurring high costs for sourcing alternatives, revalidating formulas, and updating labels. In some cases, companies had to manage product recalls or deal with stockouts due to halted distribution. These challenges highlight the critical need for companies to monitor the regulatory environment closely and develop forward-looking strategies to protect business continuity and minimize financial risk. [6] [7] [9]
The Compliance Nightmare
Managing ashwagandha regulation across multiple European markets has become a full-time challenge for supplement companies. Businesses now often need different formulations and labels. As reported by NutraIngredients [10], industry leaders describe the complexity of navigating divergent national regulations as “a logistical nightmare,” with some companies forced to manage multiple product versions for different markets.
One regulatory affairs executive at a leading supplement company explained, “We went from managing one European formula to juggling eight different versions. The complexity alone nearly broke our operations team.”
(Industry interview, 2025)
Brand Trust and Consumer Confusion
Perhaps most damaging was the impact on consumer confidence. Shoppers accustomed to buying ashwagandha online suddenly faced conflicting information about safety. Social media was filled with confused customers asking, “Is Ashwagandha safe?” and “Why is Ashwagandha banned?”
Companies invested heavily in consumer education, explaining the difference between quality standardized ashwagandha extract and potentially problematic products. Those with USP-verified ashwagandha and NSF-certified ashwagandha products found themselves with a competitive advantage.
What Steps Can You Take to Stay Compliant?
1. Build Bulletproof Documentation: RegASK Guidebooks
In a climate of increasing regulatory scrutiny, documentation isn’t just a formality; it’s your first line of defense. The most resilient companies go beyond compiling existing studies; they develop strategic dossiers that anticipate regulatory concerns.
RegASK Guidebooks expertly validates safety and compliance packages built to meet global standards. Whether you’re addressing toxicological risks, dosage thresholds, or usage restrictions, these guidebooks are designed to preempt objections, not react to them.
2. Leverage Proactive Regulatory Intelligence
Waiting for a ban to appear in a trade publication is no longer a viable strategy. The companies that stayed ahead of the curve used AI-driven regulatory intelligence to monitor emerging global risks in real time.
Using solutions like RegASK helps identify shifting regulatory sentiment before formal restrictions are enacted. This provides time to explore formulation and label adjustments, pivot market strategies, or engage regulators early. Such foresight helps companies maintain access to key markets and seize opportunities in regions with more favorable regulatory climates.
Being proactive doesn’t just ensure compliance; it unlocks competitive timing advantages in increasingly fragmented regulatory environments.
3. Adopt Ingredient Diversification Strategies
Over reliance on a single hero ingredient can quickly become a liability. When ingredients like ashwagandha faced bans or strict dosage caps, companies that had diversified their formulations were able to adapt while mono-ingredient brands were left scrambling.
Forward looking teams blend ashwagandha with rhodiola, magnesium, or other adaptogens to develop formulations that are not only effective but regulatory resilient. RegASK’s regulatory intelligence can guide you in identifying compliant ingredient alternatives tailored to your market and category.
Ingredient diversification isn’t just a safety net; it’s a product innovation strategy powered by regulatory foresight.
What to Expect Beyond 2025
As global scrutiny around herbal supplements intensifies, companies must prepare for a more structured and data-driven regulatory future. Three key trends will define the post 2025 landscape.
Global harmonization is accelerating. While botanical regulations remain fragmented today, initiatives like the WHO’s Traditional Medicine Strategy [13] signal a shift toward unified safety standards. For global brands, short term complexity persists, but the path to simplified compliance is emerging.
AI-driven compliance is becoming the industry norm. Modern regulatory teams are turning to solutions like RegASK to anticipate changes, map ingredient risks, and streamline workflows to help eliminate guesswork from global compliance.
Evidence-based claims are required. Authorities are tightening restrictions on unsupported health claims. Success will depend on clinical validation, transparency, and the ability to adapt quickly to new claims substantiation requirements by understanding local regulations and processes.
Looking ahead, compliance isn’t just about keeping up; it’s about staying ahead. The winners will be those who treat regulatory agility as a core business strategy.
如何 RegASK helps navigate Global Regulatory Complexity
RegASK simplifies regulatory decision making with an AI-driven solution that delivers timely insights, smart documentation, and workflow orchestration. From identifying risk ingredients to managing compliance across regions, RegASK empowers regulatory teams to act faster, mitigate risks timely, and stay ahead of change; globally and on a scale.
Ready to future-proof your compliance strategy? 预订演示 to see how RegASK can help you navigate regulatory complexity with confidence.
Frequently Asked Questions (FAQ)
- Why did Denmark ban Ashwagandha? Denmark banned Ashwagandha in 2023 after a risk assessment highlighted potential hormonal disruption and reproductive safety concerns. The Danish Veterinary and Food Administration required all Ashwagandha containing products to be withdrawn from the market to protect public health.
- Which other herbal supplements are facing increased scrutiny in Europe? Besides Ashwagandha, several herbal supplements are under regulatory review in Europe, including kratom, green tea extracts, chaste tree berry, red clover, black cohosh, and Dong quai. Authorities are assessing their safety, potential liver toxicity, and possible interactions with medications, which may lead to stricter regulations or bans.
- How do these bans affect global supplement companies? Global bans create significant operational challenges including product recalls, reformulation costs, country specific compliance requirements, and loss of consumer trust. Many companies are adopting regulatory intelligence platforms to proactively monitor and manage these risks.
- How can companies future-proof against changing herbal supplement regulations? Companies should invest in comprehensive safety documentation, AI-driven regulatory intelligence, diversified ingredient portfolios, and evidence-based clinical research. Platforms like RegASK help businesses anticipate regulatory shifts and maintain market access globally.
References and Citations
- sciencedirect.com – Adaptogen
- hsa.gov.sg – HSA updates on products found overseas that contain potent ingredients (March 2025)
- hsa.gov.sg – HSA Alert: Four Products Found To Contain Potent Medicinal Ingredients Including Steroids And Banned Substance; Four Consumers Had Adverse Effects After Consuming Three Of The Products
- gov.il – Counterfeit “Natural” Products
- kbvresearch.com – europe-ashwagandha-extract-market
- pmc.ncbi.nlm.nih.gov – Danish ban on Ashwagandha: Truth, evidence, ethics, and regulations
- mcgill.ca – Why did Denmark ban Ashwagandha?
- olemiss.edu – ashwagandha-khan-chittiboyina
- vitafoodsinsights.com – EU regulatory update on ashwagandha
- nutraingredients.com – Growing concerns over safety of ashwagandha in EU member states
- foodcomplianceinternational – France issues opinion on the potential adverse effects of Ashwagandha
- .bfr.bund.de – Ashwagandha: food supplements with potential health risks
- mtci.bvsalud.org – WHO convenes first high-level global summit on traditional medicine to explore evidence base, opportunities to accelerate health for all