This is RegASK
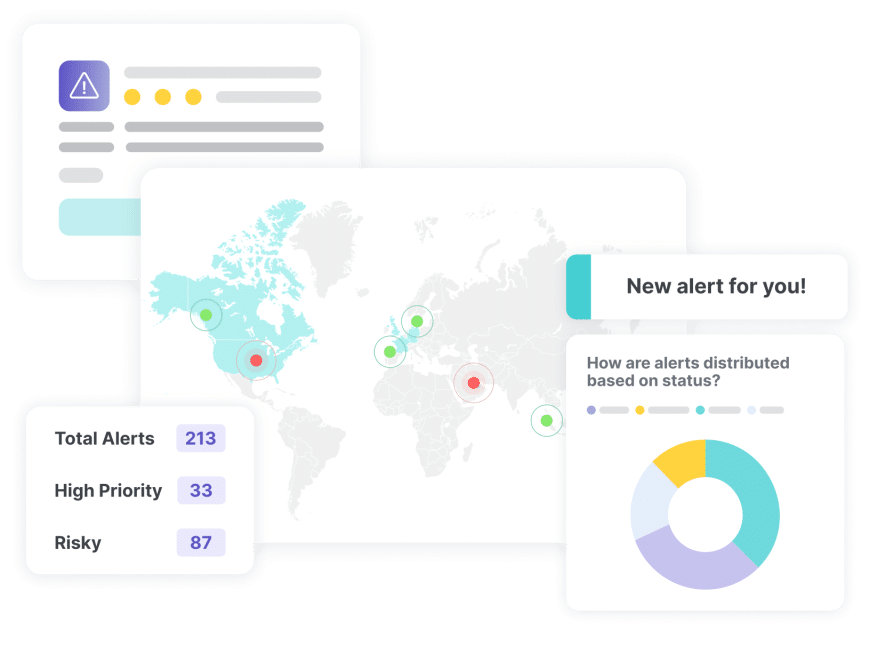
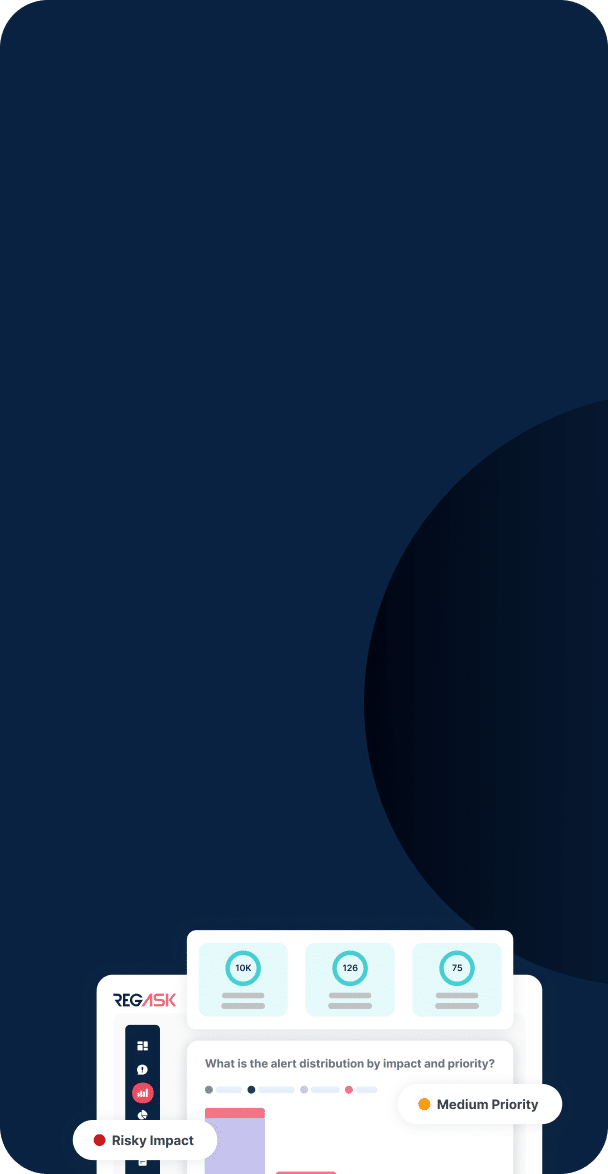
We help organizations navigate complex regulatory environments by tracking and analyzing regulatory changes, providing compliance insights, and supporting regulatory readiness.
Our vision
We build to identify your risk and increase efficiency in your product life cycle. Our augmented AI technology combined with our community of global regulatory experts, delivers unmatched efficiencies for your product workflow, and can significantly reduce your risk of noncompliance.