How are face masks regulated?
While the regulations for face masks will vary by country or region, compliance also depends on the type of face mask and the way it is marketed.
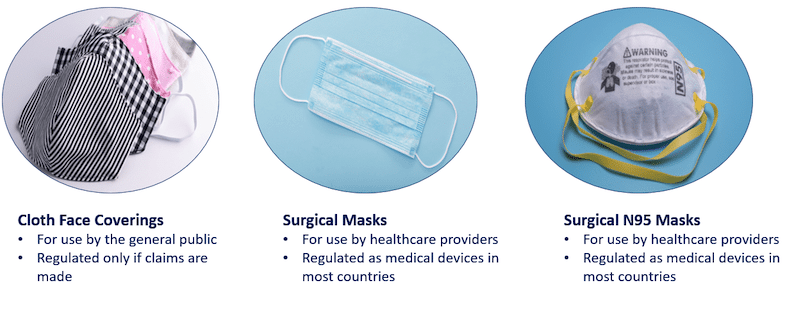
Surgical masks and respirators are regulated most often as medical devices, whereas community masks, designed for personal use, are largely unregulated.
Temporary Regulatory Guidance
China Has Increased Enforcement.
Due to an increase in counterfeit and substandard products, China temporarily imposed additional regulatory requirements on five types of medical devices to be exported, including COVID-19 test kits, surgical masks, surgical preventive clothing, ventilators and infrared thermometers.
Inversely, The United States Has Eased Restrictions.
To allow for an increase in PPE supplies, the U.S. FDA and CDC have relaxed import regulations. Masks from China may be eligible to come into the United States under an existing emergency use authorization. The FDA is also open to working with PPE manufacturers that are not currently on the market in the U.S., or with companies looking to pivot to make face masks and other PPE.
Europe’s temporary allowances have expired.
In Europe, regulatory restrictions were briefly relaxed to optimize the supply chain and allow for the influx of supplies. Most temporary allowances were in effect for 30 days and have since expired. Many European countries were affected by counterfeit products, and therefore, the EU is hesitant to ease restrictions again.
How We Helped A PPE Company Expand To New Markets
A leading Japanese medical device company wanted to expand its line of face masks to Europe during the coronavirus pandemic. The client wanted to include an on-label pollution blocking claim.
RegASK worked with its on-the-ground experts with insider knowledge of the EU to ensure product entry into the maximum number of countries possible.

