Emerging technologies like artificial intelligence, predictive analytics, and blockchain are redefining how medical devices and Software as Medical Devices (SaMD) are built, used, and regulated. Consequently, this rapid innovation brings growing complexity for regulatory teams across the globe.
This is where Regulatory Technology (RegTech) plays a pivotal role. By using tools like AI in healthcare, blockchain, and automation, RegTech is helping companies meet evolving compliance requirements while staying ahead in the market.
It is no longer just a reactive solution to compliance challenges. It is now at the core of proactive, future-ready strategies. As global agencies like the FDA, MHRA, and NMPA China update their frameworks, 8 key trends reveal how RegTech is shaping the future of global medical device compliance.
1. AI-Driven Regulatory Intelligence
Artificial Intelligence (AI) is transforming how medical device companies manage compliance. AI-driven RegTech tools now scan large volumes of data, predict regulatory changes, and give actionable insights. For Software as a Medical Device (SaMD) developers, this means quicker approvals and faster time-to-market. NLP-based platforms also simplify complex regulations across countries.
To support this shift, global regulators have taken clear steps. Below are key initiatives shaping AI compliance.
| Regulation/Authority | Impact |
|
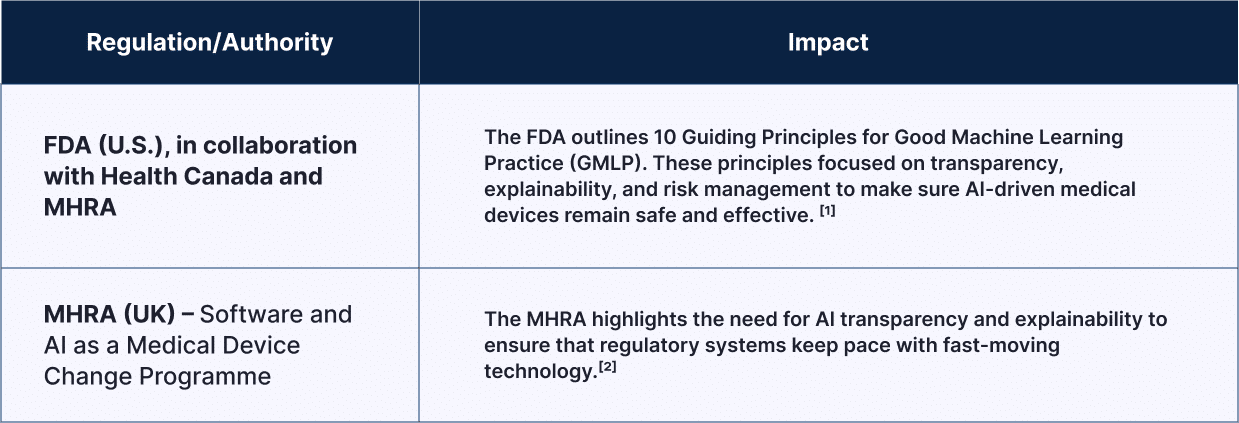
FDA (U.S.), in collaboration with Health Canada and MHRA
|
The FDA outlines 10 Guiding Principles for Good Machine Learning Practice (GMLP). These principles focused on transparency, explainability, and risk management to make sure AI-driven medical devices remain safe and effective. [1] |
| MHRA (UK) – Software and AI as a Medical Device Change Programme | The MHRA highlights the need for AI transparency and explainability to ensure that regulatory systems keep pace with fast-moving technology. [2] |
Together, these efforts build a foundation for safe and effective AI use in healthcare. They guide companies as they innovate within a clear and modern regulatory framework.
2. Real-Time Compliance Monitoring
With the rise of connected medical devices and IoT-enabled SaMD, real-time compliance monitoring is becoming critical. RegTech solutions now integrate with device ecosystems to track performance, flag anomalies, and ensure adherence to regulatory standards. This proactive monitoring reduces risk and strengthens patient safety by keeping devices within approved limits. [3]
As regulators adapt to this shift, they emphasize continuous oversight. The examples below show how major agencies are addressing real-time monitoring.
| Regulation/Authority | Impact |
| FDA (U.S.) – Draft Guidance on AI-Enabled Medical Devices | The FDA stresses the need for continuous monitoring to manage performance drift and data shifts, which helps ensure devices stay safe and effective throughout their lifecycle. |
| NMPA (China) – Renewal of Standardization Technical Committee | The NMPA emphasizes real-time compliance and post-market surveillance as key elements in managing AI-based medical devices effectively.[4] |
These regulatory actions signal a clear move toward ongoing oversight. By embedding compliance into daily device performance, companies can better manage safety, reliability, and trust.
3. Blockchain for Regulatory Transparency
Blockchain is gaining traction in the medical device space for its ability to provide tamper-proof and transparent records. From supply chain tracking to audit trails for SaMD updates, blockchain-powered RegTech tools help ensure data integrity and smoother audits. This is especially useful for SaMD, where managing software updates and version history is crucial for compliance.
As transparency becomes a regulatory priority, authorities are beginning to align blockchain with their compliance frameworks. The examples below show how global regulators are supporting this shift.
| Regulation /Authority |
Impact |
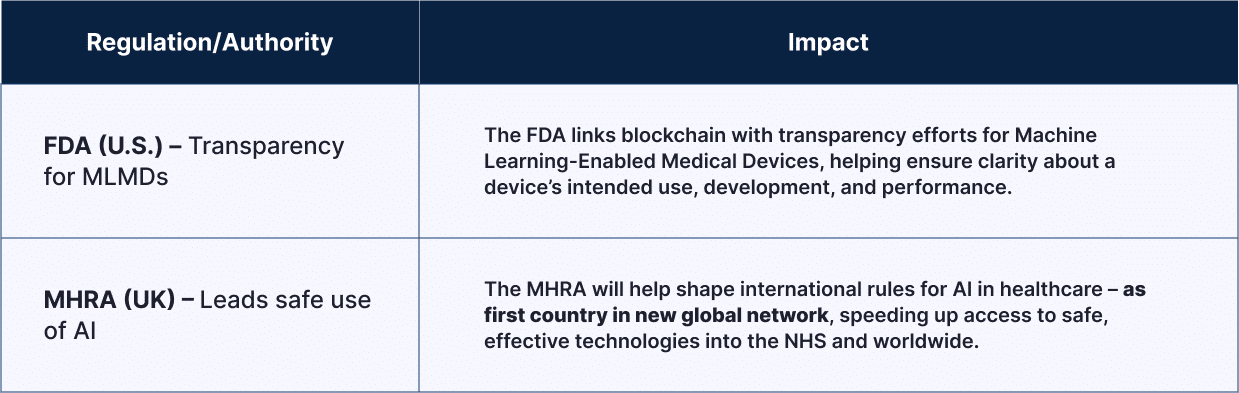
| FDA (U.S.) – Transparency for MLMDs | The FDA links blockchain with transparency efforts for Machine Learning-Enabled Medical Devices, helping ensure clarity about a device’s intended use, development, and performance. |
| MHRA (UK) – leads safe use of AI | The MHRA will help shape international rules for AI in healthcare – as first country in new global network, speeding up access to safe, effective technologies into the NHS and worldwide. |
By adopting blockchain, regulators are laying the groundwork for more open, auditable, and secure medical device ecosystems, especially for software-based products. [5]
4. Global Harmonization of Regulatory Standards
As SaMD and medical devices are increasingly launched worldwide, companies must navigate a maze of different regulatory systems. RegTech tools are now enabling unified dashboards, automated submissions, and multi-region mapping to streamline this process. These developments are helping businesses expand globally while staying compliant.
In response, regulators are building partnerships and updating frameworks to support global alignment. Below are key initiatives leading this effort.
| Regulation /Authority |
Impact on Global Harmonization |
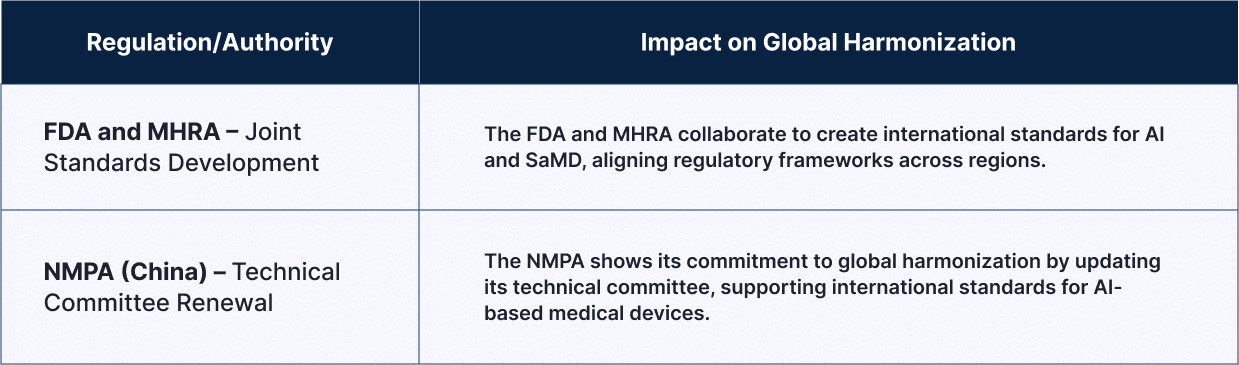
| FDA and MHRA – Joint Standards Development | The FDA and MHRA collaborate to create international standards for AI and SaMD, aligning regulatory frameworks across regions. |
| NMPA (China) – Technical Committee Renewal | The NMPA shows its commitment to global harmonization by updating its technical committee, supporting international standards for AI-based medical devices. |
These global efforts are simplifying compliance across borders and allowing safer, faster scaling of AI-enabled health technologies.
5. Predictive Analytics for Risk Management
Predictive analytics is changing how companies manage compliance risks. By using historical patterns and present data, RegTech solutions can detect threats early and recommend preventive steps. For SaMD, this means better handling of cybersecurity issues, data protection, and shifting regulatory expectations.
To support this, regulators are now integrating predictive analytics into post-market processes. Here’s how they are applying for it.
| Regulation/Authority | Impact on Risk Management |
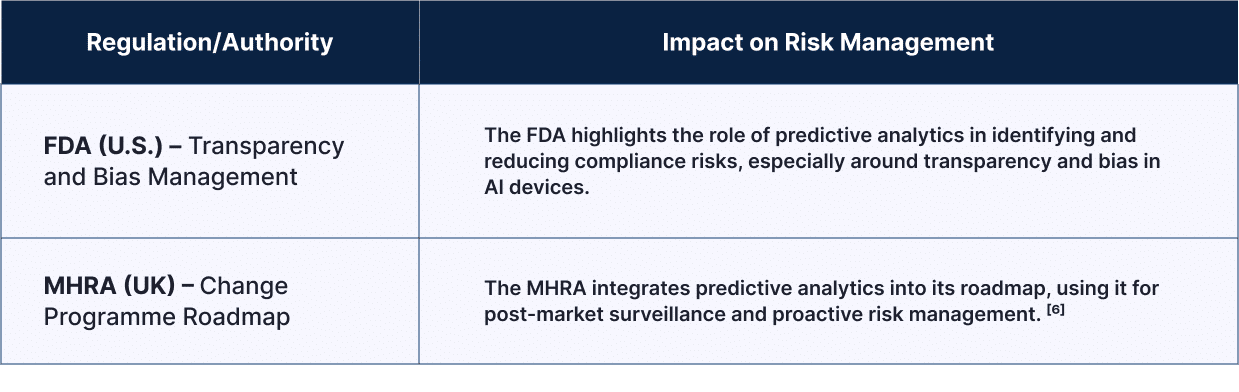
| FDA (U.S.) – Transparency and Bias Management | The FDA highlights the role of predictive analytics in identifying and reducing compliance risks, especially around transparency and bias in AI devices. |
| MHRA (UK) – Change Programme Roadmap | The MHRA integrates predictive analytics into its roadmap, using it for post-market surveillance and proactive risk management. [6] |
These steps show how regulators are using data-driven insights to build smarter and more responsive compliance frameworks.
6. Enhanced Collaboration Between Regulators and Industry
The rise of RegTech is improving how regulators and companies communicate. Digital platforms now support faster feedback loops, simplified submissions, and clearer regulatory pathways. This is especially valuable for SaMD developers, whose products evolve more rapidly than traditional devices.
Regulatory bodies are actively building systems that encourage collaboration. Here are examples of those efforts.
| Regulation/Authority | Impact on Regulator–Industry Collaboration |
| FDA (U.S.) – Digital Health Center of Excellence | The FDA encourages collaboration through this center, improving safety and deployment of AI-enabled medical devices. |
| MHRA (UK) – Software Group | The MHRA’s Software Group drives collaboration and ensures regulatory compliance for SaMD and AIaMD while supporting innovation. |
This shift is creating a more agile and open regulatory environment, allowing innovation and oversight to move forward together. [7]
7. Focus on Cybersecurity and Data Privacy
With more connected devices and AI-driven tools in healthcare, data protection is a top concern. RegTech tools are now incorporating encryption, access control, and privacy-by-design principles to meet evolving compliance demands. These advances are key to maintaining patient trust and regulatory approval.
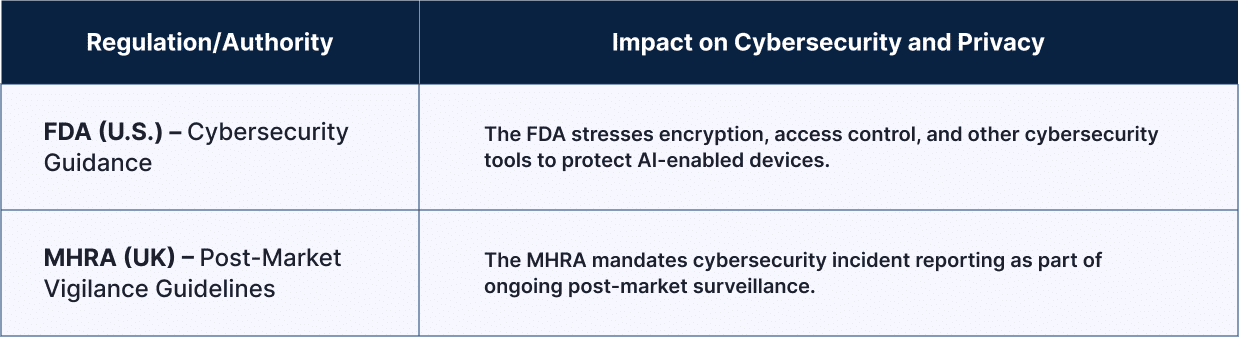
Regulators are strengthening cybersecurity rules as threats grow. The table below shows how they’re responding.
| Regulation/Authority | Impact on Cybersecurity and Privacy |
| FDA (U.S.) – Cybersecurity Guidance | The FDA stresses encryption, access control, and other cybersecurity tools to protect AI-enabled devices. |
| MHRA (UK) – Post-Market Vigilance Guidelines | The MHRA mandates cybersecurity incident reporting as part of ongoing post-market surveillance. |
These moves reflect the growing expectation for secure, privacy-conscious medical technologies throughout the product lifecycle. [8]
8. Agile Regulatory Frameworks for SaM
As SaMD continues to evolve at a rapid pace, companies and regulators are shifting toward more flexible regulatory frameworks. RegTech supports this shift by enabling timely updates, iterative testing, and continuous compliance checks. This approach ensures that new innovations stay compliant as they scale.
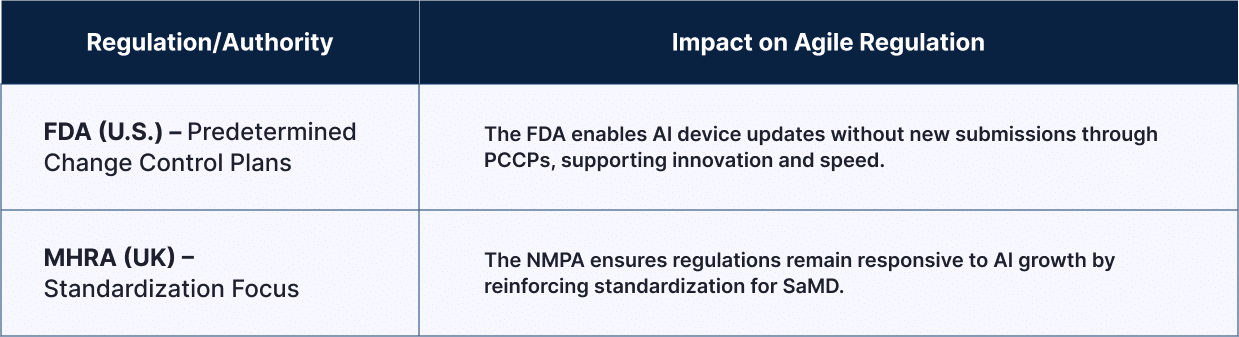
Regulatory bodies are now adopting tools and rules that support faster cycles. Below are some key examples.
| Regulation/Authority | Impact on Agile Regulation |
| FDA (U.S.) – Predetermined Change Control Plans | The FDA enables AI device updates without new submissions through PCCPs, supporting innovation and speed. |
| NMPA (China) – Standardization Focus | The NMPA ensures regulations remain responsive to AI growth by reinforcing standardization for SaMD. |
These agile frameworks mark a shift from rigid oversight to dynamic, tech-aligned regulation that supports fast-paced innovation in healthcare.
Final Thoughts
As technology continues to evolve, the frameworks that govern it must evolve too. RegTech is not just keeping pace with innovation. It is enabling it. From blockchain to predictive analytics and global harmonization, these trends show how intelligent compliance tools are reshaping the medical device landscape. For regulators and companies alike, embracing these shifts is no longer optional. It is essential for building trust, accelerating innovation, and protecting patient safety in a rapidly changing world. [9]
At RegAsk, we are committed to helping medical device and SaMD companies navigate these changes with clarity and confidence. Our regulatory expertise and advanced RegTech solutions empower you to focus on what matters most: improving patient outcomes through innovation.
Ready to lead the future of compliance?
Discover how RegASK’s RegTech solutions can help you stay compliant, competitive, and ahead of the curve in the evolving medical device and SaMD landscape.
Book a Demo with RegAsk-
Fda.gov – Transparency for Machine Learning-Enabled Medical Devices: Guiding Principles
-
Gov.uk – Software and artificial intelligence (AI) as a medical device
-
Nmpa.gov.cn – China Medical Device Standards Management Annual Report (2023)
-
Gov.uk – UK MHRA leads safe use of AI in healthcare as first country in new global network
-
Gov.uk – Software and AI as a Medical Device Change Programme roadmap
Subscribe to the latest regulatory news
Curated newsletters
Relevant industry info
Access expert insights