Empowering Smarter Regulatory Decisions
Curated AI-Driven Regulatory Intelligence
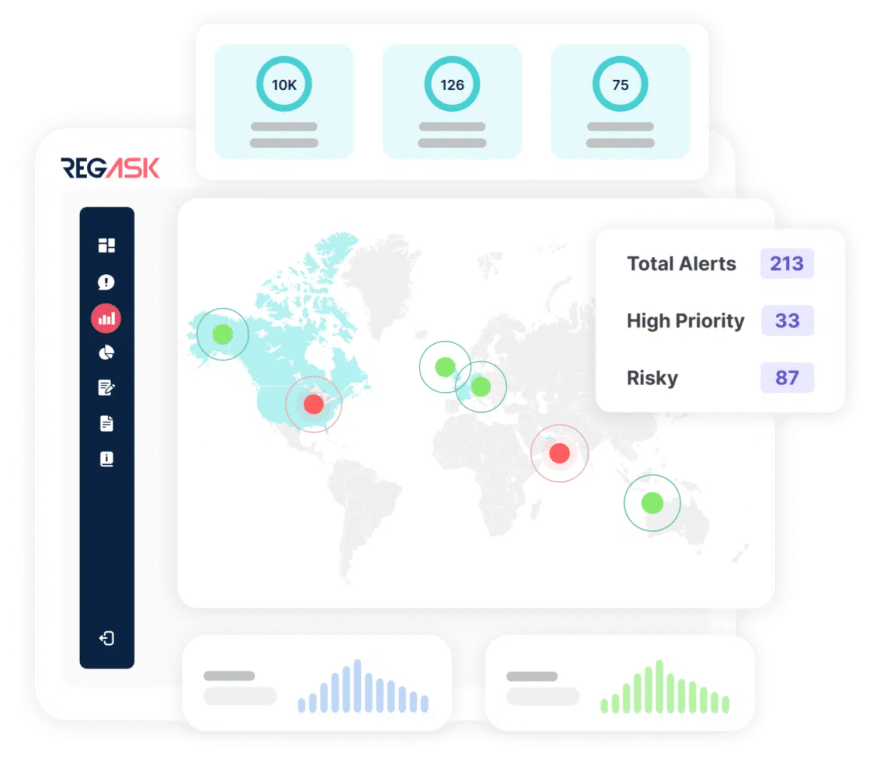
Curated AI-Driven Regulatory Intelligence

RegASK empowers organizations to navigate complex regulatory landscapes by providing actionable insights, expert guidance, and AI-Driven solutions.
Our platform and expert network streamline compliance processes, mitigate risks, and drive business growth.

“What sets RegASK apart is their practical approach that is catered to you, their flexibility to align with your strategy, and the ability to target deep expertise globally. I highly recommend RegASK. They are true partners in navigating the regulatory maze.”
Shiva Kannan
Chief Operations Officer
Fibronostics

“RegASK helped me deep dive into several Asian markets with beauty devices/skincare. This saved me 20+ hours of research and freed me to complete other projects. We had great success in registering our China skincare also. Just wonderful to work with and it is a real partnership!”
Danielle Barker Fernandes
Senior Manager R&D/ R.A.
NuFACE

“We have worked with RegASK on several specific projects which enabled us to clarify internally certain choices we needed to make based on different levels of regulatory risk across countries. This provided an important framework to structure our strategic thinking.”
Béatrice Guelet
International Regulatory Affairs Manager
NAOS
Countries
Experts
Regulatory Data Points
Publishers
Stay in the know.
Optimize workflows.
Regulatory compliance powered by AI.