We’re pleased to share that RegASK was featured in Everest Group’s Innovation Watch: AI Applications in Clinical Development 2026.
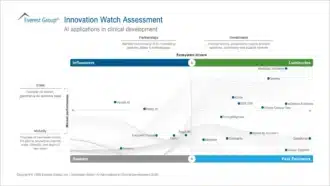
The report presents Everest Group’s Innovation Watch review of 18 leading AI providers in clinical development. It groups companies into four profiles: Luminaries, Fast Followers, Influencers, and Seekers, based on capability and market influence. It also examines provider solutions, market barriers, adoption patterns, and commercialization models. Together, these insights show where AI is already delivering real value and what to expect as these technologies mature.
Was der Artikel hervorhob
The feature places RegASK in the Seeker category within the clinical development AI landscape. This reflects strong market maturity, based on the scale, depth, and progression of use cases from pilot to production. It also highlights RegASK’s partnership strength, measured by the number and diversity of system integrator and consulting partners and its visibility across the ecosystem.
Warum das wichtig ist
Clinical development is entering a new phase shaped by AI. Processes are becoming more data heavy, more regulated, and more complex across every stage of the value chain. This report examines how generative, agentic, and advanced AI are changing the way life sciences organizations design, run, and govern clinical trials. It offers a clear view of where these technologies are already being applied, what barriers remain, and how leading organizations are preparing for what comes next.
Wie RegASK hilft
RegASKs inclusion in this report reflects the growing need for platforms that connect regulatory intelligence, organize fragmented workflows, and turn regulatory change into clear actions. In an environment where speed, compliance, and execution drive trial success, solutions like RegASK are becoming essential infrastructure for faster, safer clinical development.

