Life Science regulatory affairs professionals balance the competing demands of new regulations, emerging technologies, and ever-changing market conditions. Rising emphasis on digital health technologies, real-world evidence, and harmonization across markets present additional complexity to navigate.
At RegASK, our goal is to help life science companies navigate this complex landscape seamlessly, by leveraging technology and our global community of experts.
Contact us Book a demo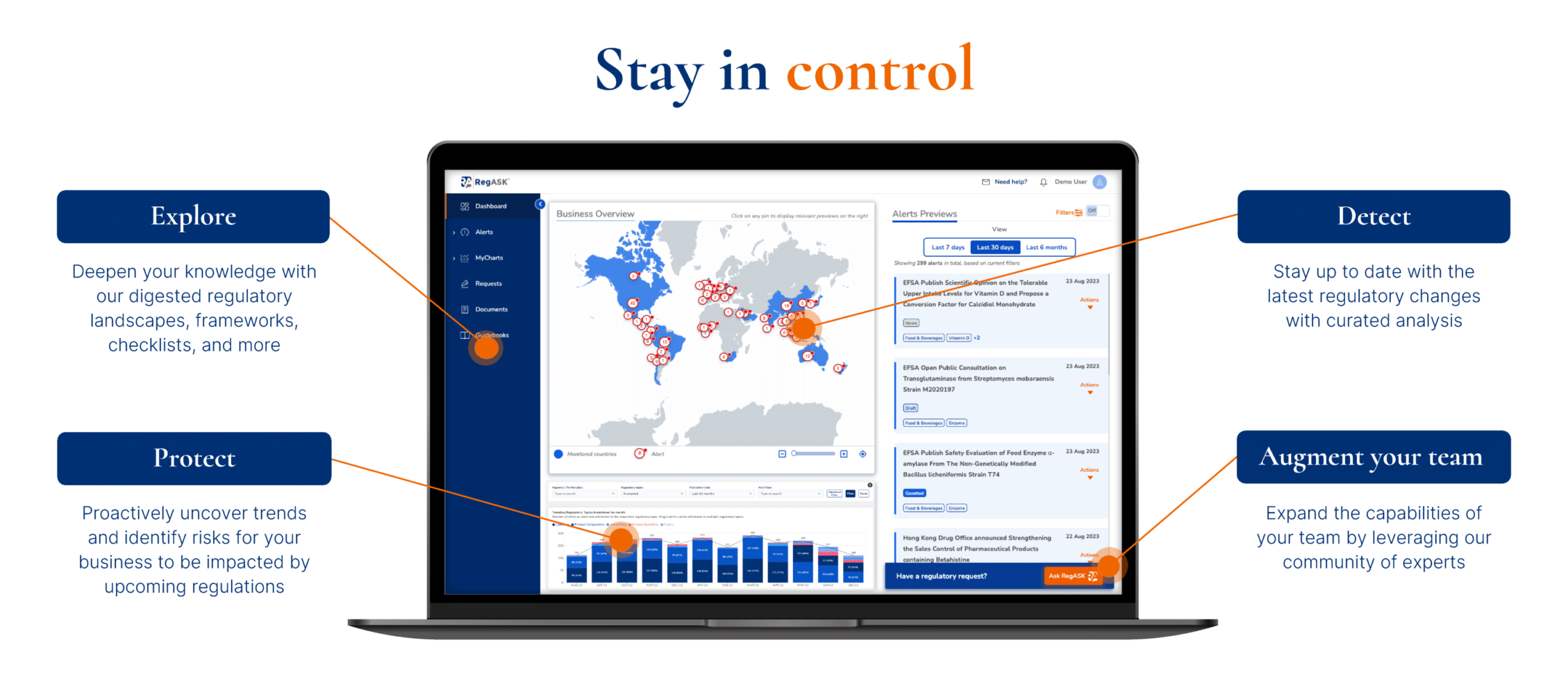
RegASK:
A combination of Advanced Technology x Human Expertise
RegASK provides tech-enabled solutions to augment companies’ regulatory capabilities and enhance process efficiency. We combine AI technology with our global network of subject matter experts to support our clients from regulatory intelligence to execution. We aim to help you bring your life-changing products and therapies to market faster with the power of RegASK.
500+
International Experts
120+
Countries
70%
Time Saved
900+
Publishers
RegAlerts
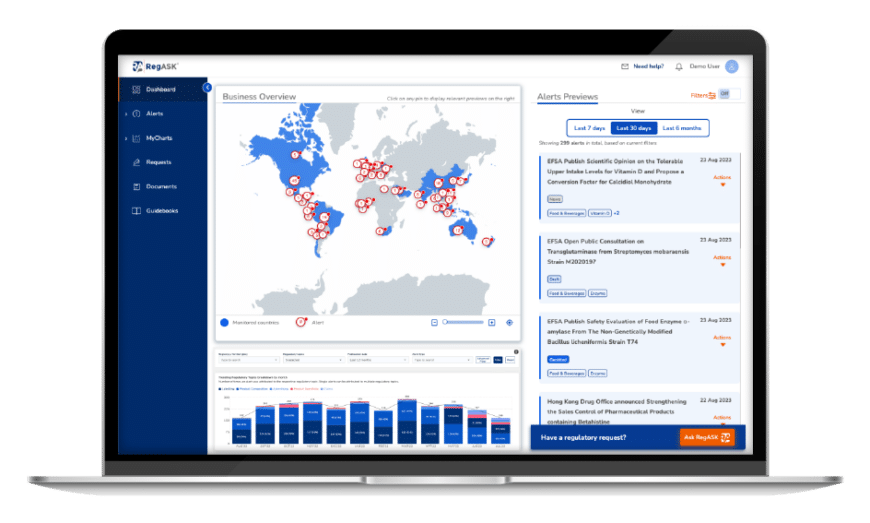
Monitor regulatory changes
Filter out the noise and focus on business–specific regulatory intelligence, powered by AI – your regulatory co-pilot.
RegInsights
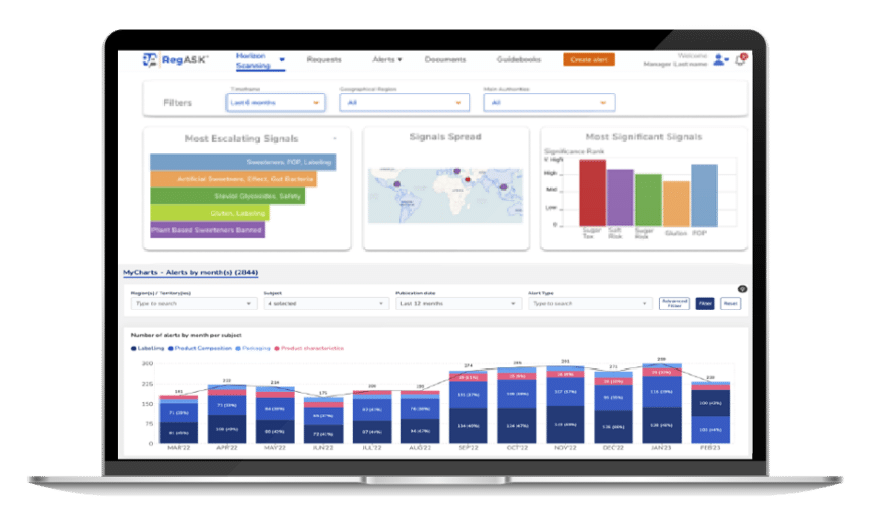
Anticipate business risks
Leverage data to proactively understand regulatory trends and opportunities impacting your products and organization.
RegGuides
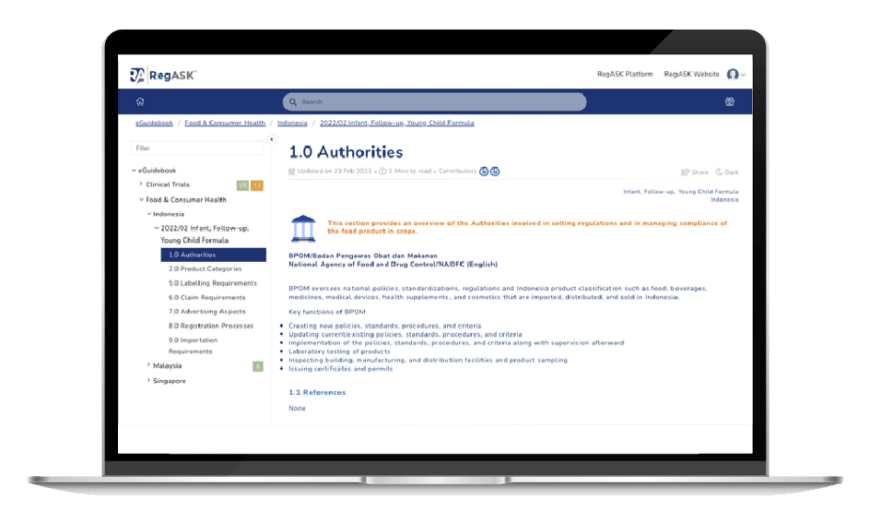
Explore curated resources
Access our library of digested current regulatory landscapes, checklists and all you need to know to go to market.
Ask RegASK
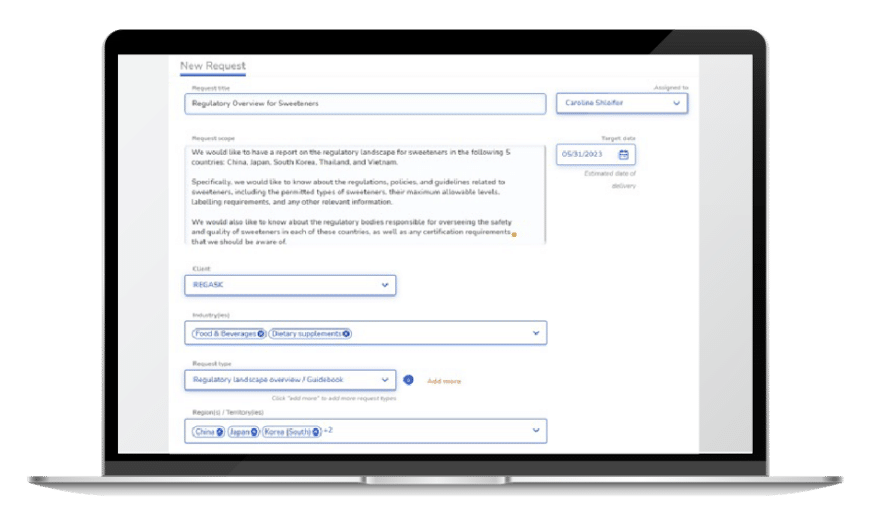
Get on-demand support
Augment your team with a global network of experts for local needs, from regulatory strategies to dossier submissions.

RegAlerts
Staying ahead in the ever-changing regulatory landscape is key to Life Sciences companies. Our AI-powered solution identifies and continuously monitors relevant sources of information (agencies, associations, news) to proactively detect and track regulatory changes from drafts of regulations, published updates, news, standards, and guidelines. It alerts your regulatory team whenever a change may impact the commercial strategies of your drug or medical device. This is designed to provide you with actionable insights to empower your decision-making process.



RegInsights
We understand the importance of staying ahead in the ever-changing Life Sciences regulatory landscape and the critical decisions needed to ensure a successful path to market. RegInsights, harnessing AI, empowers your company with data & trend analysis, providing seamless access to crucial regulatory information and actionable insights, allowing teams to identify and adapt to emerging regulatory trends effectively and ensuring streamlined compliance and informed decision-making processes.

RegGuides
Increase your knowledge and broaden your understanding of regulations that impact your Life Science business by having self-service access to curated content. Our resources serve as a go-to reference for Life Science organizations navigating complex regulatory frameworks from various countries. You will gain access to digested and actionable regulatory landscapes, synthetized compliance requirements and streamlined roadmaps and checklists of regulatory guidance documents, regulations, and directives.

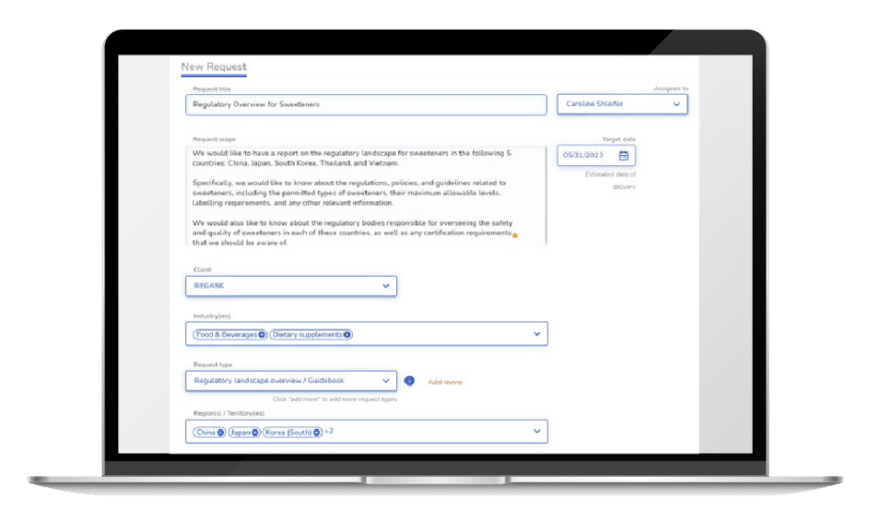

Ask RegASK
For as long as your product is on the market, we ensure compliance with local regulations. Ask RegASK, with its network of curated experts, offers 24/7 support for life science companies, ensuring ongoing compliance with local regulations and providing essential support in managing submission files and regulatory strategy discussions, relieving regulatory compliance pressures, and offering comprehensive support throughout the regulatory journey. Ask RegASK provides costs and document requirements, reviews clinical study documentation, and shares appropriate interactions with regulatory agencies during audits, inspections, and communications.

Generate, Review and Manage Submission files
- Preparation of CTA, IMPD and IND applications to support clinical trials
- Manage submissions such as NDA, BLA, ANDA, DMF, BMF, EUA, 510(k), De Novo, PMA, IDE, MDR
- Reviewing & approving product labelling, packaging, and promotional materials for compliance.
- Applications for Licensing, Pre-Clinical, Clinical Trial Study, Medical device listing, etc.

Prepare your Path to Market Regulatory Strategy
- Definition of filing pathways, costs and documents requirement
- Participating in the development of clinical trial protocols & synopsis
- Review Clinical study documentation
- Interacting with regulatory agencies during audits, inspections, and communications

Analyse Risks/Benefits for Regulatory Compliance
- Perform gap analysis and risk mitigation actions
- Assist companies for product/company certifications (i.e., QMS), etc.

Get Regulatory Support & Liaison with Agencies
- Regulatory compliance planning
- Interpretation of regulatory documents, and directive
- Review and Critique of technical documentation
- Ensuring post-market compliance through ongoing surveillance & assessment
Our AI-enabled regulatory platform and global network of local experts get you to market faster.
Request a demo today to see how RegASK can help you save resources, minimize risk, and drive patient outcomes.