In August 2022, following a six-month transition period, the EU regulatory ban on food additive E171 titanium dioxide came into full force. This new regulation was several years in the making, and RegAlert subscribers in the food, pet food, cosmetics and pharmaceutical industries benefitted from regular alerts from its very earliest stages. Our first of 17 regulatory monitoring alerts regarding the potential prohibition of titanium dioxide was published in October 2020, the last in October 2022. This allowed users to anticipate the impact of the ban on their businesses and products, explore alternative ingredients and ensure compliance when the final regulations were announced.
What is titanium dioxide?
Titanium dioxide (TiO2) is a naturally occurring mineral that is mined, then processed and refined into a fine white powder. It is used as a pigment in a wide range of consumer products, from paint and sunscreen to food coloring.
In its food grade form, titanium dioxide is known as food additive E171. As with all food colorings, the purpose of titanium dioxide is to enhance the color and visual appeal of food products, giving color where there is none, or restoring color that has been lost during processing. Titanium dioxide is also commonly used in pet food, cosmetics and pharmaceutical products.
What foods contain titanium dioxide?
Up until the August 2022 ban, titanium dioxide was used prolifically throughout the food industry. It whitened and brightened skimmed milk, ice cream, cheese, soups, sauces, salad dressings and sandwich spreads to name but a few E171-enhanced products. Its shiny white properties were also beloved by confectionary and baked goods producers, who used it in chewing gum, candy, frosting and cakes.
Need support on formulation and ingredients? Contact RegASK
Why was titanium dioxide banned as a food additive?
Titanium dioxide was first approved for use as a food additive by the European Union in 1969. In 2016 (like all other additives approved for use within the EU before 2009), titanium dioxide underwent a new safety evaluation, carried out by the European Food Safety Authority’s (ESFA) ANS Panel. The review left them unable to fix an Acceptable Daily Intake (ADI) for titanium dioxide due to concerns over particle size and the lack of research into its possible effects on the reproductive system.
ESFA scientists then screened thousands of scientific publications and assessments to better understand the potential dangers of titanium dioxide. New data on the “genotoxicity” of nanoparticles – in other words, how these particles can damage DNA, leading to cell mutations and possibly cancer – was of particular interest. Some animal studies showed how titanium dioxide nanoparticles could trigger inflammatory bowel diseases and colorectal cancer, demonstrating the potential impact on human health[1].
In May 2021, the ESFA updated its safety assessment, announcing that, based on continued uncertainty related to genotoxicity, titanium dioxide was no longer considered safe for use as a food additive[2].
This updated assessment led to the EU’s 2022 decision to ban the use of titanium dioxide as a food additive (Commission Regulation (EU) 2022/63). The new regulations came into force on 7 February 2022, with a six-month transitional period[3]. This allowed manufacturers to continue placing products made under the old rules on the shelves until 7 August 2022, where they could remain until their ‘use-by’ date.
However, the debate on the safety of titanium dioxide continues. A recent statement issued by the EU Court of Justice in November 2022 announced the annulment of the Commission Delegated Regulation of 2019, which recognized titanium dioxide as a potentially carcinogenic substance by inhalation in certain powder forms. The General Court found the classification of titanium dioxide as a carcinogenic substance was not based on reliable studies, and that proper classification criteria had not been applied[4]. While this change does not currently affect the ban on titanium dioxide as a food additive[5], it does raise questions about the scientific methodologies used to study the ingredient, and demonstrates the volatility of regulatory changes and the importance of staying up to date.
How RegAlert notified subscribers of impending and actual regulatory changes
The first titanium dioxide alert was published on the RegAlert platform on 27 October 2020. Sixteen more regulatory alerts related to the use of titanium dioxide in the food, pet food, cosmetic and pharma industries were to follow. Eleven of these were generated in the EU, UK or Switzerland:
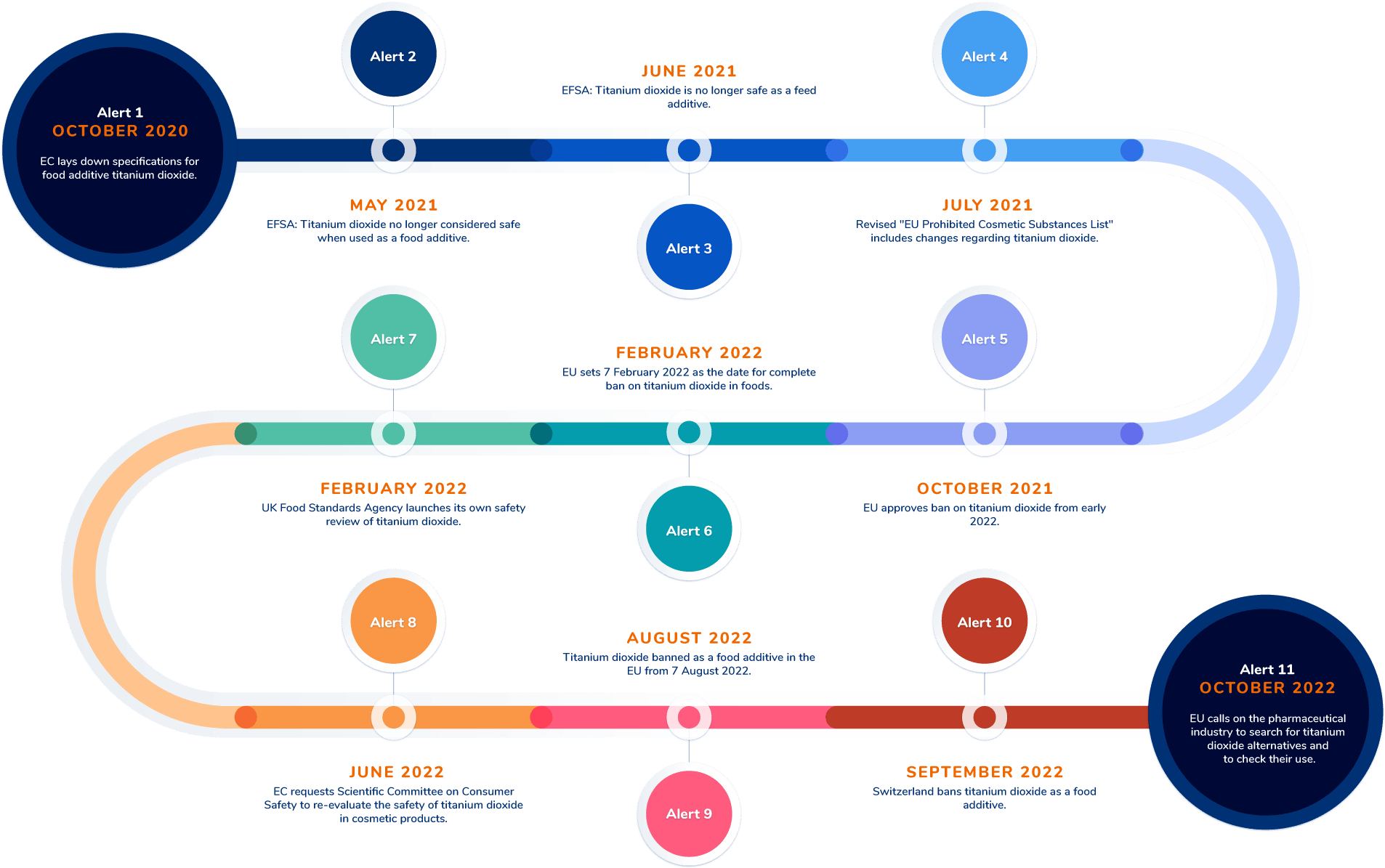
Alert 1: 27 October 2020
A horizon scanning alert for the food industry highlighting the lack of European Commission (EC) opposition to new regulations restricting the use of titanium dioxide as a food additive[6].
Alert 2: 6 May 2021
An alert noting the revised EFSA assessment of titanium dioxide as unsafe for use as a food additive[7].
Alert 3: 18 June 2021
An alert for the feed industry noting the revised EFSA assessment of titanium dioxide as unsafe for use as an animal feed additive[8].
Alert 4: 12 July 2021
The EC makes amendments to Regulation (EC) No 1223/2009 regarding the use of titanium dioxide as an ingredient in cosmetics products[9].
Alert 5: 20 October 2021
EU member states approve the EC’s decision to ban titanium dioxide as a food additive, with effect from early 2022.
Alert 6: 2 February 2022
The EU sets 7 February 2022 as the date for the complete ban on the use of titanium dioxide in the food industry. A six-month grace period allows foods produced before 7 February to be placed on the market until 7 August 2022[10].
Alert 7: 2 February 2022
A horizon scanning alert for the food industry, noting the launch of the UK Food Standards Agency’s own safety review of titanium dioxide[11].
Alert 8: 30 June 2022
A horizon scanning alert for the cosmetics industry noting the request from the EC for the Scientific Committee on Consumer Safety (SCCS) to re-evaluate whether titanium dioxide is safe for use in cosmetics products[12].
Alert 9: 1 August 2022
Alert for the food industry highlighting the end of the transitional period and commencement of the total ban on titanium dioxide in food products placed on the market from 7 August 2022[13].
Alert 10: 15 September 2022
Alert confirming food industry regulations in Switzerland now align with the EU: Titanium dioxide is prohibited in any products manufactured after 15 September 2022[14].
Alert 11: 3 October 2022
A horizon scanning alert highlighting calls by the EU for the pharmaceutical industry to search for alternatives to titanium dioxide in medicines and pharmaceutical products[15].
How can RegASK help companies detect and avoid the risk of non-compliance with ingredient bans?
The case of titanium dioxide is a great example of how industry regulatory standards are continually evolving. RegAlert’s constant monitoring of all news related to this particular ingredient has allowed subscribers to be well prepared for the changes. With horizon scanning alerts going back as far as 2020, users of titanium dioxide had ample time to adapt their strategies, research alternative ingredients, create new formulas and update packaging and labelling, ensuring their products remained compliant within the targeted markets.
Non-compliance of products in any industry can lead to full product recalls, expensive penalties, brand damage, loss of market share, and in extreme cases, even criminal charges. Using RegAlert to track updates related to all ingredients used within a product range means businesses can move forward with confidence, and avoid costly surprises.
So far, only the food industry has seen a concrete impact with the forewarned updates to regulations regarding titanium dioxide now firmly in place. This sets the scene for more regulatory changes to come. As we can see from our chain of alerts, these will most likely affect the use of titanium dioxide as an ingredient in medicinal products, cosmetics products and animal feed.
We invite companies making products for the pharmaceuticals industry, the animal feed industry and the cosmetics industry, as well as the food industry, to use our regulatory monitoring services to track updates on all their ingredients; anticipation is key and we are here to help.
Get in touch today to find out how RegASK and RegAlert can help your business stay one step ahead; be ready for the changes to regulations that will affect you, your products, your business, and your bottom line.
What you'll get with RegASK
- Early detection and mitigation of regulatory risks
- End-to-end regulatory support throughout your product lifecycle
- Strategic consulting to build the optimal business strategy for your commercial success
Book a demo
References:
[1] Common food additive found to affect gut microbiota
[2] Titanium dioxide: E171 no longer considered safe when used as a food additive
[3] Commission Regulation (EU) 2022/63 of 14 January 2022 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the food additive titanium dioxide (E 171)
[4] The General Court annuls the Commission Delegated Regulation of 2019 in so far as it concerns the harmonised classification and labelling of titanium dioxide as a carcinogenic substance by inhalation in certain powder forms
[5] Titanium dioxide still banned in Europe
[6] ANNEX to the Commission Regulation (EU) amending the Annex to Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards specifications for titanium dioxide (E 171)
[7] Titanium dioxide: E171 no longer considered safe when used as a food additive
[8] Titanium dioxide: no longer considered safe as feed additive
[9] Draft Commission Regulation amending and correcting Annex II and amending Annexes III, IV and VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products
[10] Commission Regulation (EU) 2022/63 of 14 January 2022 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the food additive titanium dioxide (E 171)
[11] EU titanium dioxide ban enters into force: what happens next?
[12] SCCS – Request for a scientific advice on Titanium dioxide (TiO2) in cosmetic products
[13] Titanium Dioxide is No Longer Authorised as a Food Additive in the EU from 7 August 2022
[14] Interdiction du dioxyde de titane comme additif alimentaire en Suisse dès l’automne 2022
[15] New Questions and Answers from the EMA regarding Titanium Dioxide in Medicines

